In the fight against infectious diseases, the first step is the correct identification of the causative agent, and the symptoms and lesions that it causes in the host. A correct diagnosis influences on the effectiveness of the treatment established, particularly if it is combined with preventive measures such as vaccination. Traditional techniques such as microscopic observation and oocysts counting remain very useful as screening methods, and an aid in the diagnosis and treatment of coccidiosis in animals. DNA-based methods such as PCR have overcome some limitations of these conventional methods, allowing the analysis of more samples in less time, increasing sensitivity and allowing the quantification of the parasite in one step.
These new methods positively influence the treatment of coccidiosis, expanding the possibilities for the poultry veterinarian to control the disease.
Diagnosis of coccidiosis in poultry by stool analysis methods in the laboratory has some limitations inherent to the type of sample used. Faeces collected on the farms are sent to the laboratory for microscopic examination of Eimeria oocysts. If this first step takes too long oocysts change their morphology, which makes microscopic identification tricky. The possibility of obtaining false results when analysing samples in poor condition is high. This may affect the effectiveness of the treatment of coccidiosis in broilers and laying hens.
Another limitation of conventional parasitology in the diagnosis of coccidiosis in poultry is the simultaneous presence of three or more species of Eimeria within a flock of birds. If the treatment of coccidiosis or its prevention by vaccination is aimed at the kind of acting Eimeria, misidentification could induce treatment failures. Finally microscopy as a diagnostic method of coccidiosis in poultry is limited to the identification of the species of parasite and the approximate amount present in a gram of sample. Other characteristics such as resistance to certain anticoccidials, etc., cannot be assessed by these techniques.
At the end of the 90s, new methods of detection and identification of Eimeria in chickens based on DNA techniques were developed. Specifically PCR (polymerase chain reaction) protocols for epidemiological purposes (Schnitzler 1998) were described. Subsequently the PCR method has been improved, and in the 2000s the first methods of real-time PCR (Kawahara 2008) that reduced analysis time, improve assay sensitivity and reduce cross-contamination between samples were optimized. This made possible to establish the treatment of coccidiosis more accurately and quickly.
The refinement of PCR methods has been continued in recent years. One of the limitations inherent to the analysed samples is the presence in the faeces of substances capable of inhibiting the action of the polymerase, which is the engine of the synthesis of DNA copies during the amplification step. The development of optimized methods of obtaining and purifying DNA (Gerhold 2015), and the addition of internal control (IPC) in each reaction well (Raj 2013), has largely reduced false negative results due to reaction inhibition. The IPC must be positive in each and every one of the samples analysed in order to be considered a valid PCR assay.
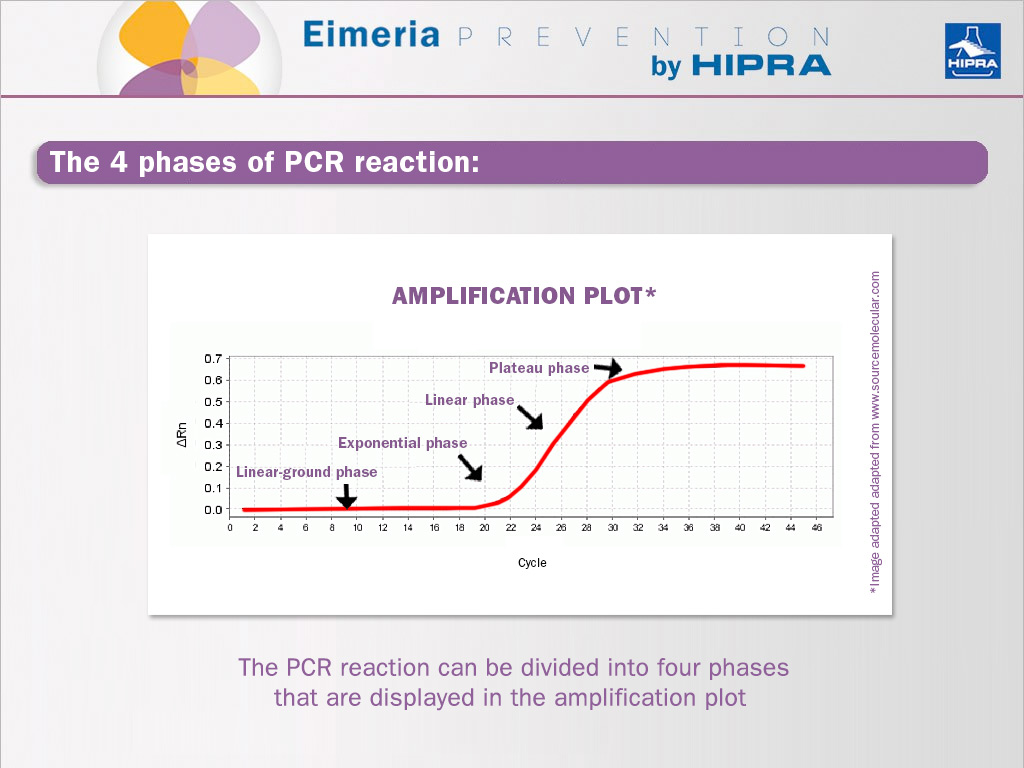
The PCR reaction can be divided into four phases that are displayed in the amplification plot (see image above).
More recently, the real-time PCR has been optimized for simultaneous obtaining of qualitative and quantitative results. Thus, it is possible to get accurate identification of the species of Eimeria present in the sample as well as the amount of each of them (Nolan 2015). At present, the PCR is a robust and target-specific detection method for Eimeria in poultry. The PCR solves some major limitations of microscopic examination of stool, allowing analysis of large numbers of samples in less time, the examination of faeces, tissues and even suspensions of oocysts, and even serves as a method to ensure the identity and purity of strains present in vaccine preparations (You 2014).
Therefore, the PCR has a direct impact on the treatment of coccidiosis, based on a timely and reliable diagnosis. However the laboratories using quantitative PCR methods for the diagnosis of coccidiosis in poultry, require highly trained personnel, calibrated instruments, and standardized protocols to ensure repeatability and accuracy.
Finally, successes in diagnostic and subsequent treatment of coccidiosis in poultry depend largely on the interpretation of the results. Interpretation should be based on the clinical history, the estimated time of infection and signs and lesions observed. PCR along with the classic method of observation of the parasite and the clinical judgment allows improving the treatment of coccidiosis on the basis of a comprehensive approach.
This video animation illustrates the main advantages of the quantitative real time PCR.
REFERENCES:
- Schnitzler B.E., Thebo P.L., Mattsson J.G., Tomley F.M., Shirley M.W., 1998. Development of a diagnostic PCR assay for the detection and discrimination of four pathogenic .Eimeria species of the chicken. .Avian Patholology 27:490-7.
- Kawahara F., Taira K., Nagai S., Onaga H., Onuma M., Nunoya TKawahara F., 2008. Detection of five avian Eimeria species by species-specific real-time polymerase chain reaction assay. Avian Disease 52(4):652-6.
- Gerhold R.W., McDougald L.R., Beckstead R.B., 2015. A novel, simplified technique to amplify Eimeria (Coccidia: Apicomplexa) DNA from oocysts. Journal of Parasitology. 101 (1):102-3.
- Raj G.D., Aarthi S., Selvabharathi R., Raman M., Blake D.P., Tomley F.M., 2013. Real-time PCR-based quantification of Eimeria genomes: a method to outweigh underestimation of genome numbers due to PCR inhibition. Avian Patholology 42 (4):304-8.
- Nolan M.J., Tomley F.M., Kaiser P., Blake D.P., 2015. Quantitative real-time PCR (qPCR) for Eimeria tenella replication — Implications for experimental refinement and animal welfare. Parasitology International 64 (5):464-470.
- You M.J., 2014. Detection of four important Eimeria species by multiplex PCR in a single assay. Parasitology International 63 (3):527-32.




