Immunity against coccidiosis disease is tightly linked to the parasite life cycle. It is a complex cycle, with a combination of exogenous and endogenous stages that trigger a response by the host’s immune system.
However, Eimeria parasites have also been described as being highly elusive to the immune system, as well as producing chemokines that can slow or inhibit the host’s immune response (Jang 2011, Schmid 2014, Miska 2013). Eimeria spp. replication occurs inside intestinal cells; therefore, it has been proposed that the cell-mediated immune (CMI) response, regulated by type 1 T helper cells (Th1 immunity), plays a major role in protecting birds against coccidiosis infection (E. del Cacho 2011 and E. del Cacho 2012).
Th1 cells produce cytokines such as interferon-γ (IFN-γ), tumour-necrosis factor-α (TNF-α) and interleukin-2, that drive CMI responses. The functional effectors of CMI responses are several immune cells like cytotoxic T lymphocytes (CD8+) and natural killer cells (NK), besides macrophages. Cytotoxic lymphocytes and macrophages are specialized against internal and external antigens, respectively.
Coccidiosis immunity is species-specific, not being cross-protection between different species. Even different strains of the same species have a varied immunogenicity. Therefore, selecting the right strains to include in a coccidiosis vaccine is essential to get a good immunity.
Three consecutives infections and replications of wild or vaccine strains are needed to develop a protective immunity against Eimeria spp.
Enhancement of immune response after coccidiosis vaccination by the use of an adjuvant
Although it is well known that live vaccines can induce adequate immunity against Eimeria spp. without being combined with an adjuvant, it is hypothesised that administering a coccidiosis vaccine together with the right adjuvant increases the vaccine’s efficacy, owing to the adjuvant’s capacity to polarise the immune response towards a Th1 response (Dalloul 2005).
EVALON® and EVANT® are both HIPRA´s live vaccines against avian coccidiosis; EVALON® is intended to protect breeders and laying hens and EVANT® is aimed to give protection to broilers. They are composed of attenuated strains of different species of coccidiosis parasite (Eimeria acervulina 003, E. brunetti 034, E. maxima 013, E. necatrix 033 and E. tenella 004 in EVALON®; Eimeria acervulina 003, E. maxima 013, E. mitis 006, E. praecox 007 and E. tenella 004 in EVANT®).
HIPRAMUNE T® is an exclusive solvent for both coccidiosis vaccines, EVALON® and EVANT®, which, between other characteristics, includes in its composition a Montanide-type adjuvant.
HIPRA firmly believes that immune modulation is crucial in providing strong, fast and long-lasting immunity against coccidiosis. So that is why HIPRA´s vaccines against Eimeria spp., as well as including strains that have been selected to maximise immunogenicity and minimise the side effects of Eimeria parasites, they are administered in combination with the adjuvanted solvent HIPRAMUNE T®.
Studies about the effects of a Montanide-type adjuvant on the immune response triggered by a coccidiosis vaccine
Study 1
In order to study the mechanisms by which an adjuvant could modulate the immune mechanisms that are triggered in the host after vaccination, some studies were performed, vaccinating birds with a live attenuated coccidiosis vaccine (EVALON®) at one day of age.
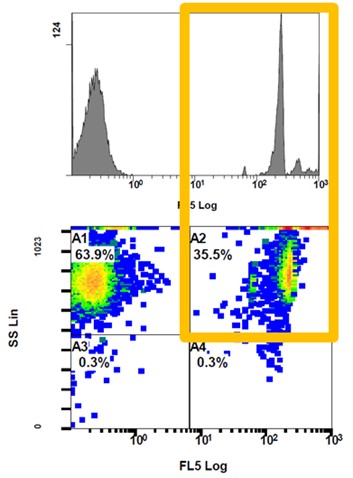
In the first trial, four treatment subgroups received EVALON®, in combination with or without the selected adjuvant, HIPRAMUNE T®, to study important markers of Th1 and Th2 responses. In birds that did not receive the adjuvanted solvent, it was replaced by a phosphate buffered solution (control group). Five birds from each subgroup were used to obtain intestinal lymphocytes from the mucosa and Peyer’s patches at different time points post-vaccination (7, 23 and 43 days p.v.). The lymphocytes were then incubated in appropriate medium and stimulated overnight with Eimeria whole antigen. Later, the lymphocytes were fixed and stained using monoclonal antibodies marked with fluorescein and studied with flow cytometry to detect lymphocytes producing IL-2, IFN-Ƴ, IL-4 and IL-10 (see Figure 1).
Figure 1. Flow cytometry images show detection of lymphocytes stained with IFN-Ƴ monoclonal antibody. Graphic representation (top) of positive lymphocytes and program output (bottom) where square A2 indicates the percentage of IFN-Ƴ-positive lymphocytes.
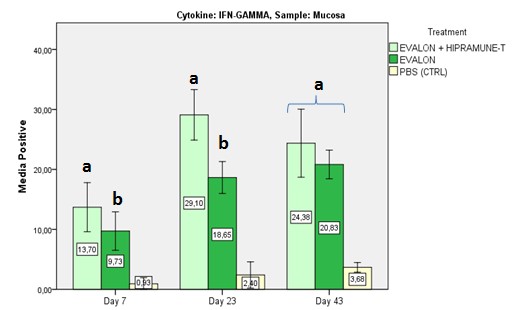
The results obtained in the first experiment indicated that HIPRAMUNE T® is able to increase the level of Th1 cytokines, as indicated by the results obtained for IL-2 (Figures 2a and 2b. Significant differences were detected at days 23 and 43 for the mucosa and at day 43 for the Peyer’s patches) and IFN-Ƴ (Figures 3a and 3b. Significant differences were detected at days 7 and 23 for the mucosa and at days 23 and 43 for the Peyer’s patches).
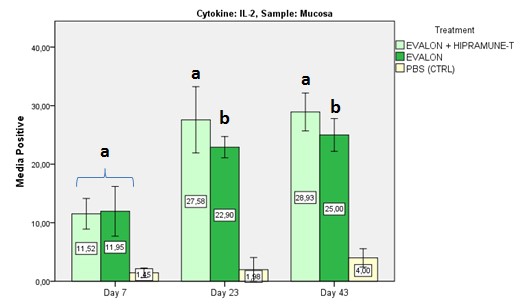 Figures 2a and 2b: Mean percentage of positive results for IL-2. Results obtained with lymphocytes isolated from the mucosa and intestinal Peyer’s patches. Superscripts (a, b) indicate statistical differences.
Figures 2a and 2b: Mean percentage of positive results for IL-2. Results obtained with lymphocytes isolated from the mucosa and intestinal Peyer’s patches. Superscripts (a, b) indicate statistical differences.
Figures 3a and 3b: Mean percentage of positive results for IFN-Ƴ. Results obtained with lymphocytes isolated from the mucosa and intestinal Peyer’s Patches. Superscripts (a,b) indicate statistical differences.
By contrast, the levels of IL-4 and IL-10 at days 23 and 43 were equal or lower when EVALON® and EVALON® + HIPRAMUNE T® were compared. These results, combined with the results recorded for IL-2 and IFN-Ƴ, confirm HIPRAMUNE T®’s ability to stimulate a cellular immune response (CMI). Therefore, it is hypothesised that EVALON® administered together with HIPRAMUNE T® is able to polarise the immune response towards a Th1 response with more intensity, as the study indicates, than the live vaccine without the adjuvant. As indicated, the Th1 response is crucial for protection against Eimeria spp.
Study 2
In a second study to assess the ability of adjuvanted solvent HIPRAMUNE® T to provide a more extended protection throughout the Eimeria life cycle, the duration of immunity (DOI) of EVALON® was studied under laboratory conditions in facilities that impaired the introduction of external coccidiosis oocysts, and that do not favour reinfections. The DOI was evaluated during 60 weeks.
EVALON® was administered via coarse-spray together with the adjuvanted solvent HIPRAMUNE® T to 1-day-old chicks. A control group was inoculated with PBS. At 14, 28, 40 and 60 weeks post-vaccination a group of vaccinated and control birds were inoculated with separate Eimeria challenges using heterologous reference challenge strains (Figure 4).
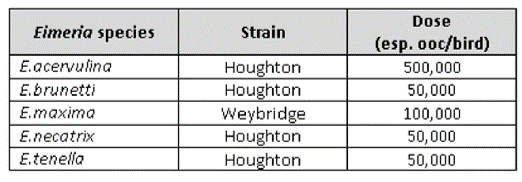 Figure 4: Eimeria strains used in challenge.
Figure 4: Eimeria strains used in challenge.
Parameters studied included: clinical signs, faeces aspect, mortality, OPG from fresh and litter faeces, weight evolution, lesion scoring post-challenge, elimination of oocysts post-challenge.
For all the challenges performed, vaccinated groups showed a reduction in lesion scores (Figure 5), oocyst excretion and clinical signs compared to control groups. Weight evolution post-challenge was not affected in vaccinated animals.
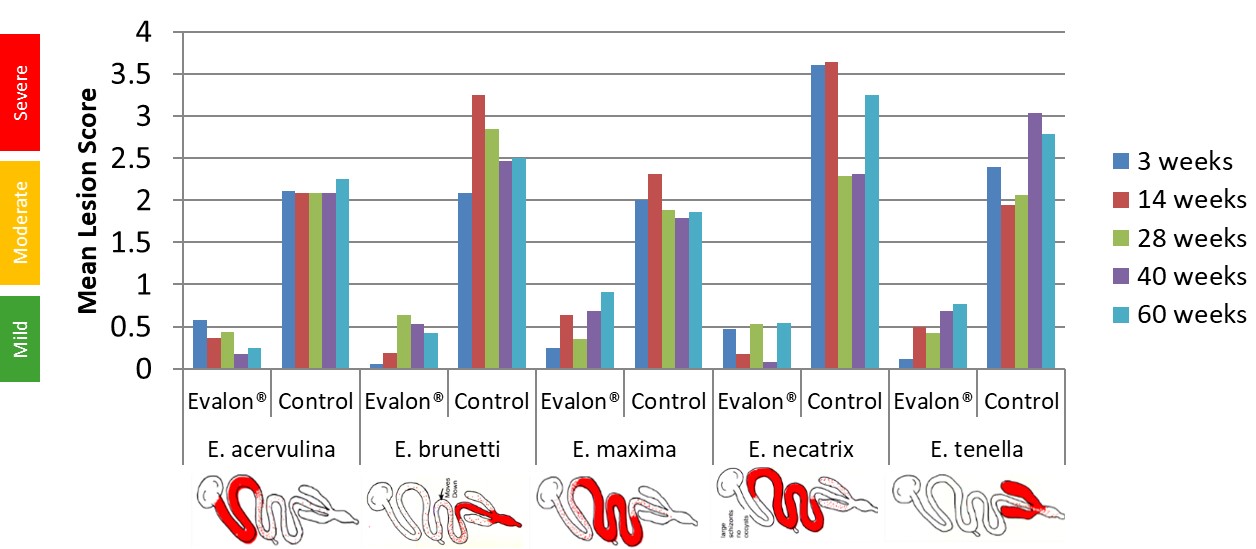 Figure 5: Results of post-challenge Lesion Scoring.
Figure 5: Results of post-challenge Lesion Scoring.
Then, duration of immunity (DOI) of the vaccine EVALON® for all Eimeria species included in the vaccine was confirmed to be until 60 weeks.
We can conclude that this extended duration of immunity in conditions that do not favour reinfections is due to the fact that EVALON®’s efficacy is boosted by co-administration with the adjuvanted solvent HIPRAMUNE T®.
REFERENCES:
- Bech G., Ros M., Sitjà M., March R., Pagès M., (2015) Extended duration of immunity in a new live coccidiosis vaccine (EVALON®) for breeders and layers with the use of an adjuvanted solvent (HIPRAMUN®E T). Proceedings of the 19th World Veterinary Poultry Association Congress. Cape Town, South Africa, 158.
- Dalloul, R.A., Lillehoj, H.S. (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Diseases 49(1): 1-8.
- Del Cacho, E., Gallego, M., Lee, S, Lillehoj, H.S., Quilez, J., Lillehoj, E., Sánchez-Acedo, C. (2011) Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine 29: 3818–3825.
- Del Cacho, E., Gallego, M., Lee, S., Lillehoj, H.S., Quilez, J., Lillehoj, E., Sánchez-Acedo, C. (2012) Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infectious Immunology 80: 1909–1916.
- Jang, S.I., Lillehoj, H.S., Lee, S.H., Kim, D.K., Pagès, M., Hong, Y.H., Min, W., Lillehoj, E.P. (2011) Distinct immunoregulatory properties of macrophage migration inhibitory factors encoded by Eimeria parasites and their chicken host. Vaccine 29(48): 8998-9004.
- Miska, K.B., Kim, S., Fetterer, R.H., Dalloul, R.A., Jenkins, M.C. (2013) Macrophage migration inhibitory factor (MIF) of the protozoan parasite Eimeria influences the components of the immune system of its host, the chicken. Parasitology Research 112(5): 1935-1944.
- Pagès M., Bech G., March R., Sitjà M., Lillehoj H., Dardi M., Rubio J., del Cacho E. (2015). Live coccidiosis vaccine for breeders and layers (EVALON®) immune modulation and enhancement of immunity by the use of an adjuvanted solvent (HIPRAMUNE® T). Proceedings of the LII Symposium Científico de Avicultura AECA-WPSA. Málaga, Spain, 199.
- Schmid, M., Heitlinger, E., Spork, S., Mollenkopf, H-J., Lucius, R., Gupta, N. (2014) Eimeria falciformis infection of the mouse caecum identifies opposing roles of IFNƳ-regulated host pathways for the parasite development. Mucosal Immunology 7(4):969-982.