When it comes to the technical aspects of the application of a coccidiosis vaccine for chickens, it is normally questions such as the composition of the vaccine that are raised (number and type of species that it contains, form of attenuation of the strains, etc.) whilst another fundamental aspect is forgotten: the route of administration.

Coccidiosis vaccine for chickens: How many application methods are there?
In the last few years, different methods have been developed for the administration of a coccidiosis vaccine for chickens (Williams, 2002). Initially, this type of vaccine was administered mainly via:
- Drinking water
- Spray on to the feed
- Eye drops: the suspension of oocysts is applied directly into the eye. In this way, the oocysts cross the nasolacrimal duct, reaching the intestine via the oropharyngeal route. This method has virtually fallen into disuse nowadays, especially in broilers, because it is so laborious.
On the other hand, as regards spray vaccination on to feed, it should be pointed out that when this route is used, it should be borne in mind that the vaccines contain sporulated oocysts in an aqueous solution.
Because of this, between the high temperatures on the first day of life on the farm and the fact that the feed is an absorbent medium, there is a high risk that the oocysts will be inactivated because of desiccation.
Administration via spray cabinets in the hatchery is a method of application to day-old chicks that has broadly replaced the methods described above.
With the use of this method, the oocysts contained in a coccidiosis vaccine for chickens are suspended in a solution with a colouring agent which has the dual advantage of allowing the effect of the vaccination on the boxes of chicks to be visualised and also of stimulating the chicks to take up the vaccine dose by preening and pecking themselves and each other.
The same route of administration can be achieved by using portable machines for smaller numbers of chicks, either in the hatchery or directly on the farm just when the chicks arrive and when they are still in the boxes.
Another method of application is the incorporation of the coccidiosis vaccine for chickens into a coloured gel that is placed in the boxes of chicks in the hatchery itself or, with the use of special equipment, it can be applied in the form of “rain” that is deposited over the chicks.
Finally, the most recent advance is “in ovo” inoculation into 18-day embryonated eggs.
“In ovo” administration, which up to now has only been feasible with a vaccine product that is not attenuated for precocity, has various advantages, including precision in application and repeatability of vaccine delivery.
Vaccination systems and equipment for a coccidiosis vaccine for chickens
a) Drinking water
When a coccidiosis vaccine for chickens is applied using this system, the most important thing is to have a sufficient number of drinking points and it is highly recommended that these should be supplemented with chick drinkers.
The drinker that permits the best delivery is the bell-type drinker with one drinker for every 100-150 chicks.
In some cases, pipette systems have been used, although this is not the appropriate conduit: the risk of the oocysts becoming trapped in the interior biofilms or in bends in the circuit is high.
There is the added risk of the different weights of the oocysts.
This means that some remain on the surface of the body of water and some at the bottom.
It is therefore likely that the bird will not ingest the oocysts of all the species contained in the vaccine.
b) Spray on to feed
For this method of administration, portable backpack sprayers are used.
The administration of a coccidiosis vaccine for chickens on to the feed requires particular care in order to avoid the desiccation of the vaccinal oocysts.
In fact, in order to achieve the same level of immunity, a larger number of oocysts per vaccine dose would be necessary, assuming that some of them would be lost by desiccation as a result of this type of administration.
c) Spray gel
Special equipment is needed for this method of application, with suitable nozzles to distribute the gel droplets over the chicks.
If the spray cabinet nozzles were used, they would soon become blocked. In addition, the preparation of the vaccine solution with the gel can be rather laborious as the gels come in the form of powder that has to be reconstituted with water with the use of a blender.
Apart from this, the distribution of the different species of Eimeria may not be homogenous in a semi-sold medium such as a gel and also, as the number of gel droplets that can be generated is lower compared to an aqueous-based spray, the opportunities for the chicks to peck the drops containing the coccidiosis vaccine for chickens are also reduced.
d) “In ovo” administration
For “in ovo” administration, the different systems that are already on the market and were originally developed for the application of viral vaccines (Marek, gumboro, etc.) can be used.
These machines have evolved to be able to deposit the vaccine inside the amniotic cavity of the embryo (more appropriate route than the allantoic cavity).
e) Coarse spray
At the present time, the most widely used equipment globally for the oral application of a coccidiosis vaccine for chickens is spray cabinets in the hatchery.
These cabinets must be able to administer the necessary droplet size for the correct ingestion of the vaccine dose and to distribute the product uniformly so that is covers the greatest number of chicks.
Normally, these cabinets are placed on conveyor belts for boxes that are already in the hatcheries, but there are also portable versions.
Key points for the correct oral administration of a coccidiosis vaccine for chickens via coarse spray
In order to ensure a uniform development of immunity in all the vaccinated chickens, there are some key points which need to be controlled:
- Correct dilution of the vaccine: any coccidiosis vaccine for chickens needs to be diluted with water in order to reach the volume of vaccine suspension corresponding to the number of chicks to be vaccinated.
This parameter is clearly linked to both the spray volume per box recommended by the vaccine manufacturer and the number of chicks contained in each box. HIPRA has an on-line dosifier calculator to calculate the final volume of the vaccine suspension of its vaccines for coccidiosis. - Droplet size of the vaccine solution: In order to be sure that the vaccine is ingested and to avoid rapid desiccation when a very fine spray is used (as in the case of spray cabinets for IB), the droplet size should be between 200 and 300 microns.
- Uniformity of distribution of the vaccine inside the box of chicks: In order to ensure that the onset of immunity is more or less simultaneous in all the chicks, it is essential that during vaccination, a large percentage of chicks in the box is reached by the vaccine solution and that they are able to ingest this solution when pecking and preening themselves and each other.
This is strictly dependent on the volume that the machine sprays per box: for a box of 100 chicks, 28 ml is the most appropriate volume to achieve good coverage of the whole box. - Colouring agents/aromas: In order to achieve correct application, it is always beneficial to add a colouring agent to stimulate the chicks to peck and take up the vaccine.
Certain aromas, under conditions of low light intensity, will also help to improve the uptake of the droplets. To find out more about the studies that HIPRA carried out for the development of its colouring agents and aromas, please take a look at this post.
Following the administration of a coccidiosis vaccine for chickens, how can we check that it has been applied correctly?
1. Directly in the hatchery, by assessing the visual effect of uniformity thanks to the colouring agent:
In the picture on the left, we can see an example of good uniformity of vaccination as there is a good number of chicks that are wet and have been reached by the colour.
This means that almost all these chicks have been reached by the vaccine and therefore, they are able to start the replication of the vaccinal oocysts.
In contrast, in the picture on the right we can see poor uniformity of application as there is a good number of chicks that are not coloured.
2. Collect samples of 10 individual fresh faeces:
Individual analysis of the faeces makes it possible to assess the uniformity of the vaccination that is the most important parameter to be taken into account.
Although the sample size may appear small, the probability of finding individual faeces without the presence of vaccinal oocysts amongst all those present in a poultry house is statistically significant.
The best ages for the collection of individual faeces for this purpose is 14 days of age.
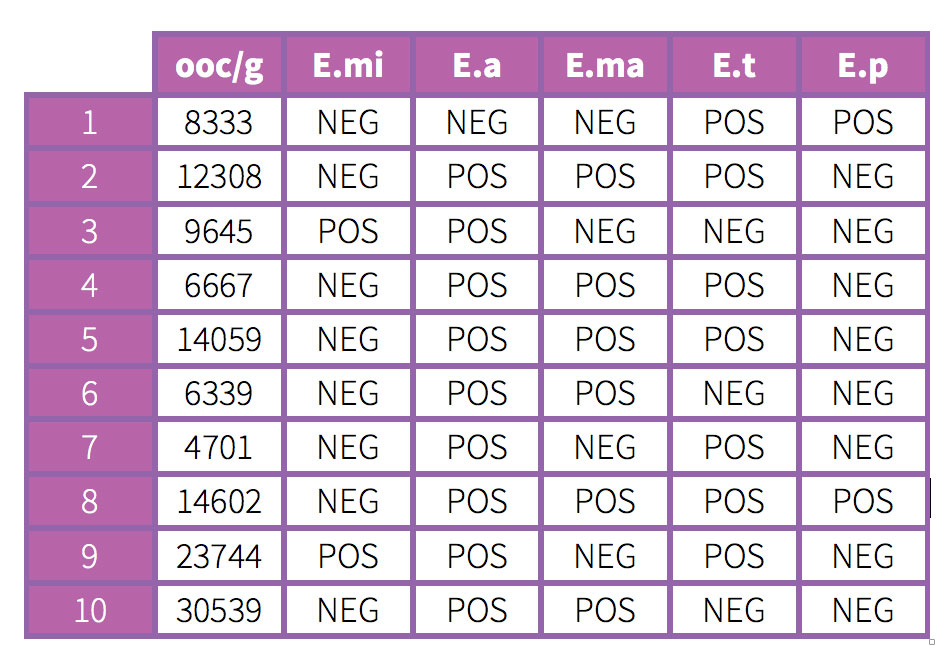
The above table shows that the10 animals have oocyst replication and that the 5 species of Eimeria contained in the coccidiosis vaccine for chickens that was used have been found amongst the 10 fresh faeces.
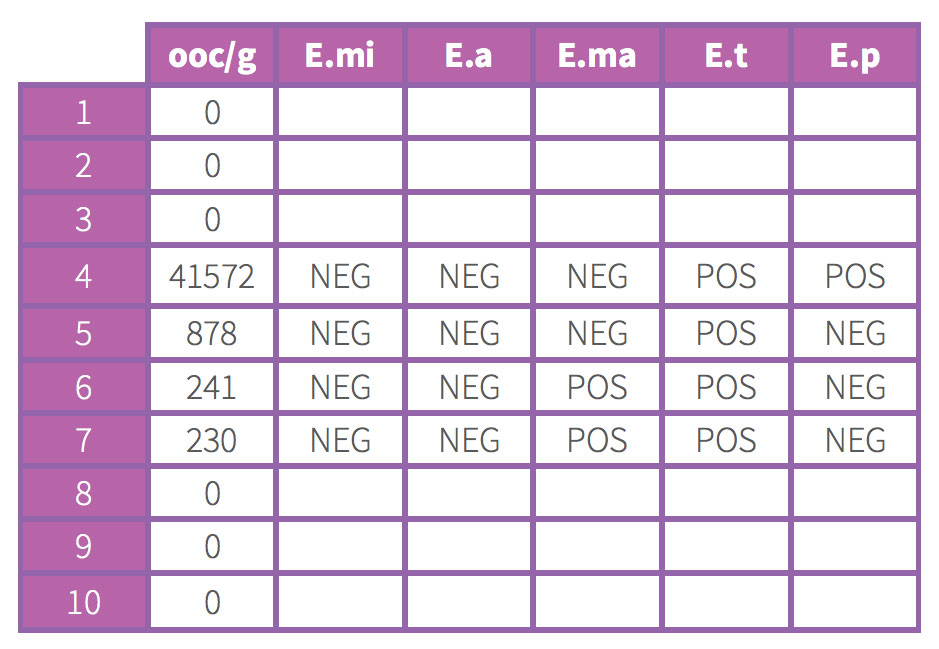
On the other hand, the second table shows poor uniformity with only 4 animals (out of 10) showing replication and with only 3 species out of the 5 present in the vaccine product.
In general, for all vaccines, a fundamental step for the successful establishment of immunity is correct application. In the case of a coccidiosis vaccine for chickens, this rule is even more important.
BIBLIOGRAPHY
- Williams R.B., 2002. Anticoccidial vaccines for broiler chickens: pathway to success. Avian Pathol. 31 (4), 317-353.




