Prevention of coccidiosis in poultry relies on live vaccines. Ionophores act as coccidiostasts by maintaining a certain level of contact with the oocyst parasite but vaccines are the only products that are able to generate a good level of specific immunity from the very beginning. Oocyst production in itself is a challenge for those companies producing vaccines
Vaccines have been considered as a method of control of coccidiosis since the early 1950s when the first products appeared. In view of the complexity of the immunity established to fight against the disease, it is necessary to work with the infective part of the parasite, i.e. the sporulated oocyst. This has been the mode of action of coccidiosis vaccines. The first vaccines were produced from pathogenic oocysts gathered in the field.
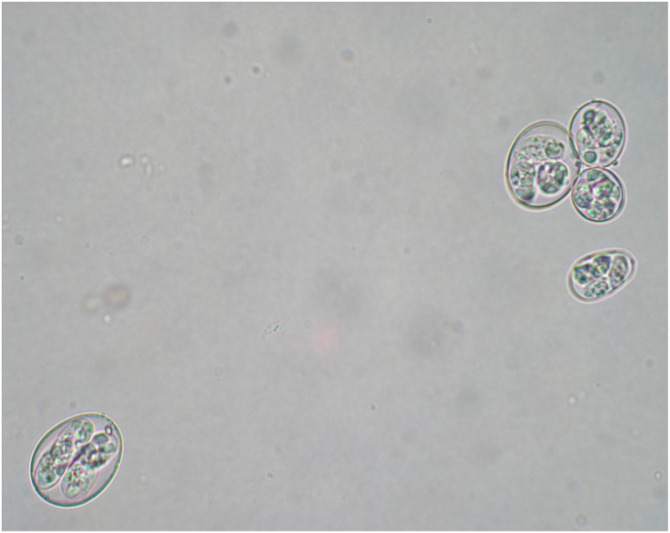
Microscopic picture (x400) of the oocysts of 5 species included in HIPRACOX® vaccine.
The oocysts were roughly processed to obtain a suspension containing a minimum quantity of sporulated oocysts. Obviously, at this time it was quite difficult to obtain a uniform number of these oocysts and the process of sporulation could not guarantee the number needed for complete immunization.
The current systems of production of vaccines against coccidiosis are based on oocyst production in live birds. A certain number of companies are producing these oocysts in SPF birds (Specific Pathogen Free) to be sure that no maternal antibodies can cause interference in the process and to minimize microbiological or viral contamination. For those who are familiar with poultry, oocyst production could be considered similar to egg production but instead of eggs, oocysts are produced. So a farm with extremely strict biosecurity measures is needed.
The main challenge of a coccidiosis vaccine is the transformation of faecal material into a sterile product and not all the vaccines marketed worldwide achieve this. This means that the process must be strictly monitored from the beginning (including the incubation of the SPF birds) to the end of the process when the vial is filled with the oocyst suspension.
Vaccines are made of oocysts of different Eimeria species. The type of species depends on the level of protection required in short or long cycle birds. The number of oocysts per species depends on the strain of Eimeria selected. This is the reason why different vaccines with the same species of Eimeria can have different numbers of oocysts per dose.
In the 1990’s, a new generation of vaccines appeared on the market. These were vaccines produced with attenuated strains. An attenuated strain is produced from an oocyst modified to generate less impact on the intestinal mucosa with a better and earlier immune response.
To produce vaccines with attenuated strains is a costly process due to the lower reproductive potential of these modified strains. However, the behaviour of these strains is totally different when comparedwith wild strains, with minimum impact on the intestinal mucosa and no impact on performance in birds.
Finally, the latest generation of vaccines includes not only oocysts but new adjuvants that are able to enhance specific cellular immunity. Based on the study of the substances that play a role in coccidiosis immunology, it is possible to identify those that are more important for activation of the cells involved in the global process and amplify their production. They could in fact be regarded as intelligent vaccines because not only do they trigger the immune system but they also help the global mechanism, especially in a disease in which cellular immunity plays an important part.



